FDA Approves VA-Made 3D-Printed Hearing Device for South Carolina Veteran
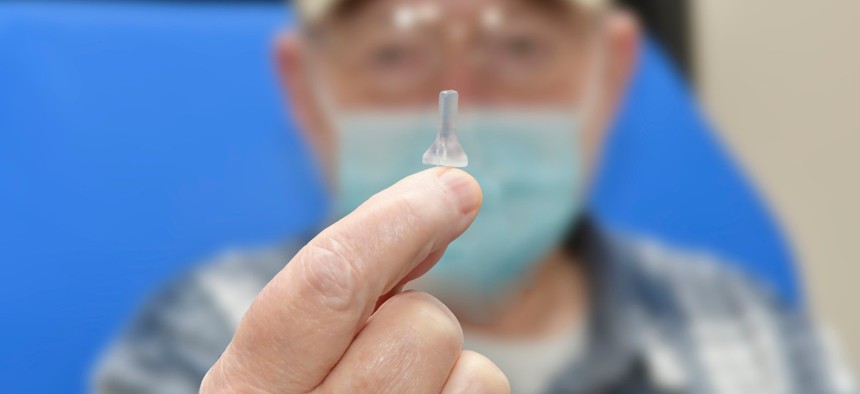
Patient Michael Nicoletti holds up the stent he helped co-design with Veterans Affairs officials. Michael Romeo III/Ralph H. Johnson VA Medical Center
It’s rooted in the patient’s unique idea to use a drinking straw to open up his hearing pathway.
The Food and Drug Administration recently approved the Veterans Affairs Department’s request to prescribe an experimental, 3D-printed device developed in-house to a 76-year-old patient facing a rare form of hearing loss.
“This was collaborative care. There are many illnesses, you know. For example, I have lung issues and heart issues and liver issues—I mean, I have more issues than issues have issues. But, in this particular case, I could participate in the solution,” that veteran patient, Michael Nicoletti, told Nextgov Monday. “And they were very open to that. They wanted my input. They wanted my feedback.”
The move marks the first time FDA granted compassionate use authorization to VA. Moving forward, the department will continue to leverage its 3D-printing network to address patient-specific medical needs that might not be met yet by off-the-shelf products. In separate interviews this week, Nicoletti, and VA’s Dr. Beth Ripley, briefed Nextgov on how the investigational, audiological device came to be, the infrastructure that made it possible and what might follow.
“3D printing—I think the majority of what it does is allow us to make individualized treatment, when we never had that opportunity to do it before,” Ripley explained. “And I think we're going to just keep growing with that.”
Grasping at Straws
Gradually over the last few years, Nicoletti—a Vietnam War veteran who lives in Summerville, South Carolina—began to recognize a clear degradation in his hearing.
“My wife and I actually noticed it here at home,” he explained, adding that as they were watching TV he began to see that she would set the volume around 20, toward the lower end of the spectrum, but he would need to set it up to 50 to really be able to hear it. “I said ‘oh boy, there’s really something wrong.,’” he remembered.
"Of course, I visit the VA,” Nicoletti noted. There, he learned he was facing conductive hearing loss, meaning the problem was a result of sound not being able to reach his inner ear like it could in the past due to blockage, in his case, caused by cartilage collapse.
Nicoletti has an engineering background and spent more than 30 years of his life designing medical equipment. One night in his kitchen, he was struck by an idea.
“I noticed we had a little container of straws. I pulled a straw out of it and I cut off about an inch of it—and I stuck that in my ear,” he said. “The docs would go crazy because I did that, but anyway, all of a sudden now I'm sitting there with the TV volume at 20 or 25—instead of 50. And I'm saying, ‘Well, there's the problem.’”
At the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, where he receives care, Nicoletti was told he might need to have surgery to mitigate his hearing issue. An operation in his 70s was not appealing in any way to the patient, so he opted to share his straw-based epiphany regarding a potential device to essentially open up his collapsing ear canal with Charleston VAMC Audiologist Kent Flanagan.
“He was excited about the idea, and so we kicked it around,” Nicoletti said, and then Flanagan brought in Biomedical Engineers Nicole Beitenman and Bethany Baldwin to transform the notion into reality. “The great thing is that these young ladies have available to them what they call a ‘3D printer,’ which they can create these devices in a matter of minutes,” he noted.
Through the Veterans Health Administration’s integrated 3D-printing network, the officials could use the medical facility’s relatively recently gained tech-based resources to create a custom straw-inspired device. Innovators across the department involved in that network have been leveraging the advanced printers at multiple sites in recent years to help confront a range of veteran-centered issues. So in South Carolina, the patient and personnel all “sat around in a huddle” to plan out a concept for the hearing tool, Nicoletti explained.
Over several months, he and the VA officials collaboratively refined and perfected the instrument “honing it to the point we're at now, where we think we have a viable device to open up that canal, without going through the pain and anguish of surgery,” he said. Currently, that device is essentially a 3D-printed stent that can be inserted into his ear canal and open it up so that sound can reach deep into it.
After iteratively producing the stent and getting it just right, Nicoletti recently took a hearing test wearing it. The patient said, during it, audiologist Flanagan “jumped for glee because he was so excited about” the notable change in his ability to hear with the device.
There are many perks to it being 3D-printed, including ease of use and an ease to replace it if an original is lost or broken, since the agency has a digital file on hand and can recreate the device when needed. Led by VHA 3D Printing Network Director Dr. Beth Ripley, the federal insiders applied for FDA’s compassionate use authorization, which enables specific, individual patients to prototype unique treatments outside of clinical trials when there aren’t comparable alternative therapy options.
“Allowing access to this Experimental 3D Printed Audiological Device is the only practical way to provide a satisfactory non-surgical solution for this patient’s collapsed ear canals to help mitigate the conductive hearing loss that it causes and improve his quality of life,” FDA wrote in a letter to Ripley approving Nicoletti’s use of the stent, which was shared with Nextgov this week.
“This is our first time applying for a compassionate use—and the FDA was really fantastic,” Ripley said, noting that there was a lot of back and forth dialogue, and “they turned this around in 14 days.”
To her, it’s an early-but-promising result of VA’s newly developed system to help facilitate such creations of patient-specific 3D-printed devices.
Just the Beginning
A radiologist by training, Ripley interprets medical images, like x-rays or ultrasounds, for diagnosis and treatment planning.
“3D printing has been a long-time love and really an extension of that for me,” she explained, noting her aims to revolutionize patient care by bringing those two-dimensional images into the 3D space. Beyond pre-surgical planning, assistive technology devices, prosthetics, and now patient-matched devices, she said there’s “a whole entire world that's been opened up to VHA through 3D printing.”
Patients often bring their own ideas to the table for fixing personal ailments, but Ripley said two “somewhat serendipitous” developments over recent years helped VA pinpoint out to bridge the divide between simple concept and wearable device.
The first was the incorporation of 3D printing into many of its hospitals, allowing frontline staff to innovate, prototype, iterate and design things that normally they could never make. A few VA facilities had been using the emerging technology by 2017—but that year, the department’s intent to establish a fully integrated 3D-printing network really came to fruition. That network has expanded to 60 hospitals since then, with VA’s printing sites almost doubling amid the COVID-19 pandemic as the manufacturing tools could help address medical supply chain issues impacting the nation.
“And the next really key element for us is that, through the pandemic, we’ve accelerated our efforts to bring three VHA hospitals into the regulatory space through registration with the FDA, and incorporation of a quality management system, which is basically kind of a whole philosophy on on how to design, test and make safe devices,” Ripley explained. “So, 3D printing, plus this registration with the FDA meant that—for the first time—when that veteran showed up in the office and said, ‘I have an idea,’ not only were the staff able to say, ‘hey, I can make it and prototype it,’ but we could also take it that next step and say, ‘hey, we can now reach out to the FDA, tell them about this device, and what we want to make—and actually be able to make it in-house under the appropriate quality guidance.’”
Accelerated by the global health emergency, that move came around the fall of 2020, when FDA required official registrations for the production of nasal swabs, of which there was a big need.
“So, that kind of pushed us over that edge, right, to like just jump in and do it. And again, like I said, it's serendipity in a way, because now that we've done that we’ve realized that now, you know, we're a manufacturer. We’re doing it. We can manufacture other devices—and what better device to manufacture than the one we could never buy?” Ripley explained. “Those single devices that may seem trivial to a large-scale, third-party manufacturer, but are essential for our patients. And so those are the types of devices that I really think you're going to see VA concentrating on in the next few years.”
In the nearer term, Ripley said VA insiders will work to continue to build out the new system and focus on training the broader workforce to employ 3D-printing assets.
“We're really excited about bringing this technology to more veterans,” she noted, “and that means we need more staff that know how to do it.”
Network officials are also still hashing out the best patient-centered use cases applicable in this space. It’s all so new that the department does not yet have a formal intake process to follow when a veteran has a concept for a 3D-printed treatment, though Ripley said they’re trying to rapidly translate what they’ve learned in these first few months into a more robust pathway for innovation.
“I think 3D printing has had a really profound impact on patient care—and everything we do is really patient-specific,” she said.
And while this initial, first approval only affirms Nicoletti’s specific treatment, Ripley confirmed that the department is now working with FDA to eventually expand its use to more veterans who might need it. A bit of groundwork must be done before it can be authorized as safe for a larger population of patients.
The original patient approved to wear it would like to see that happen.
“I can't say enough about the treatment that I received from [the VA team], and the interest, and their kindness,” Nicoletti said. “And now my hope is that, certainly it works for me, but I'm hoping that—in talking to the audiologist, he indicates there are a number of people every year that come in with the same issue—this is a solution for them. So, other vets can get their hearing back.”






