NASA Engineers Rapidly Create Ventilator Tailored to Treat COVID-19 Patients
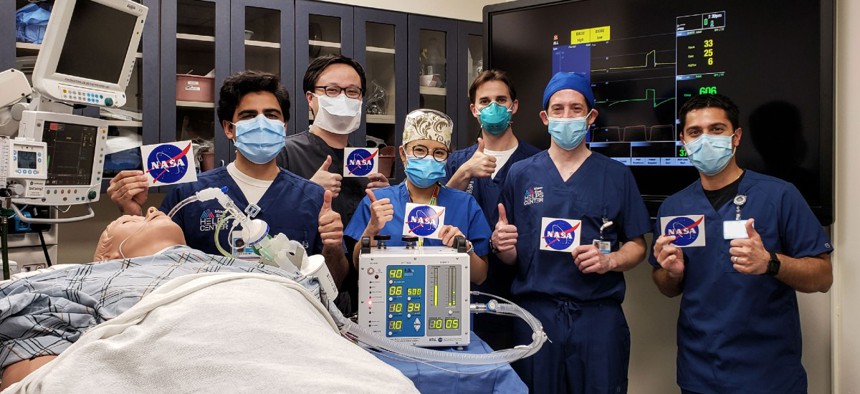
At left, doctors at the Icahn School of Medicine at Mount Sinai in New York City give a thumbs up after testing a ventilator prototype developed by NASA’s Jet Propulsion Laboratory in Southern California. Icahn School of Medicine at Mount Sinai
The new device is under Food and Drug Administration review for emergency-use authorization.
Engineers at NASA’s Jet Propulsion Laboratory rapidly produced a new high-pressure ventilator specifically targeted to treat COVID-19 patients with milder symptoms, so that America’s limited stock of traditional ventilators can be spared for those experiencing the sickness more severely.
The device—deemed VITAL, or Ventilator Intervention Technology Accessible Locally—passed a crucial test Tuesday and the agency unveiled Thursday that it is under review by the Food and Drug Administration for a fast-track emergency use authorization.
"We specialize in spacecraft, not medical-device manufacturing," JPL Director Michael Watkins said in a statement. "But excellent engineering, rigorous testing and rapid prototyping are some of our specialties.”
Watkins said the lab’s insiders “realized they might have what it takes to support the medical community and the broader community” and saw it as their duty to help out. Dozens of engineers came together for the effort, and they were able to construct the new device swiftly—in only 37 days. In a video detailing the work, NASA engineers reflected on how the “crazy project” has been both exciting and exhausting.
“We have the potential to save human lives—people that we might know, our neighbors, our families—and that intensity is amazing,” Mechatronics Engineer Michelle Easter said. “As stressful as it’s been for everybody in the last couple of weeks, not one of us can stop.”
Standard hospital ventilators are meant to last for years and be used for a wide range of medical issues, but VITAL is only expected to last three to four months. The new device is also made of “far fewer parts” than traditional ventilators, and most of its pieces are available to potential manufacturers so that the regular ventilator supply chain is not disrupted by its creation. VITAL can undergo modifications for use in field hospitals based in convention centers, hotels and other high-capacity facilities.
"Intensive care units are seeing COVID-19 patients who require highly dynamic ventilators," Dr. J.D. Polk, NASA's chief health and medical officer, said. "The intention with VITAL is to decrease the likelihood patients will get to that advanced stage of the disease and require more advanced ventilator assistance."
After initiating a range of internal testing, NASA sent the VITAL prototype to the Human Simulation Lab in the Icahn School of Medicine at Mount Sinai in New York, which is located in one of America’s hottest spots for the virus.
The high-fidelity human simulation lab’s Director Dr. Matthew Levin said the medical team was “very pleased” with their testing results. "The NASA prototype performed as expected under a wide variety of simulated patient conditions,” he explained. “The team feels confident that the VITAL ventilator will be able to safely ventilate patients suffering from COVID-19 both here in the United States and throughout the world.”
NASA’s Technology Transfer and Corporate Partnerships Office aims to offer a free license for the new device. Officials are currently reaching out to the commercial medical industry to find manufacturers for VITAL.
In a separate announcement launched Thursday, the space agency also revealed that officials at its Armstrong Flight Research Center recently collaborated with several public partners to create “an oxygen helmet to treat COVID-19 patients exhibiting minor symptoms and minimize the need for those patients to use ventilators.” Insiders this week asked the FDA to approve the specialized helmets for emergency use authorization, as well.






