Get Ready for Dissolvable Brain Sensors
agsandrew/Shutterstock.com
They can measure pressure, temperature and much more before being safely absorbed into the body.
“I just took out a bullet from the back of a guy’s head an hour ago,” says Rory Murphy.
As a neurosurgeon at the Washington University School of Medicine, Murphy “deals with brain trauma all the time.” Between bullets, blunt forces, and blood clots, traumatic brain injuries kill around 50,000 people in the United States every year. These kinds of injuries often cause the brain to swell, which constricts the flow of blood and oxygen, and can lead to permanent damage.
So surgeons like Murphy need reliable ways of monitoring the pressure inside their patients’ skulls. Sensors exist, but they are large, clunky, and must be removed once the patient has recovered.
Together with a team of engineers, Murphy is developing a better option: a dissolvable pressure sensor. Thinner than the tip of a needle, it can be left in a patient’s brain to take accurate readings for several days, before completely disappearing. You don’t need to remove them because there’s nothing to remove. They just get absorbed into the body.
These dissolvable sensors come from the lab of John Rogers from the University of Illinois. He specializes in creating flexible electronics, including electric socks for the heart, temporary tattoos that double as medical sensors, and curved cameras based on the eye.
For all these products, Rogers shunned fancy materials like graphene and used classic, cost-effective ones like silicon, instead. He simply played with the thickness and structure of these materials, so that rather than rigid, brittle wafers, he ended up with bendy, floppy sheets.
In 2012, he used similar tricks to make “transient electronics” that disintegrate in water after a given time. A silicon chip will do that anyway, given enough water and a few millennia. But by printing circuits thinly enough, Rogers can speed up that process to a matter of hours or days. That would be great for making medical implants that are only needed temporarily. For example, Rogers created a dissolvable heater that could burn away a bacterial infection in mice, before spontaneously vanishing once the animals were healthy.
When Murphy read about these electronics, he was enthralled.
“I thought they would be perfect for what we do,” he says.
Together, the duo refined Rogers's original designs into a clinically useful pressure sensor. It consists of a membrane made from PLGA, a polymer regularly used in medical devices, suspended in a frame of silicon and magnesium. The pressure of the surrounding fluid causes the membrane to bend, which changes the electrical resistance of an adjoining silicon sensor.
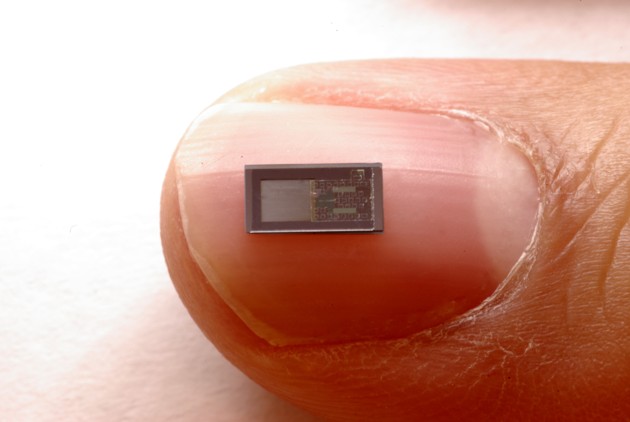
The whole device is then wrapped in a watertight polymer that gradually erodes over a few days, setting the lifetime of the sensor. (In their earlier work, Rogers’s team used silk; that’s impractical here, since silk absorbs water and would swell, pushing against the membrane in the sensor.)
When Murphy implanted the device in rats, he found that it’s as accurate as the best pressure sensors on the market. It’s also cost-effective, since it uses traditional materials with no precious metals. And most importantly, it seems to be safe. It didn’t trigger any inflammation or immune responses while it was intact, or after it had dissolved.
Nor should it.
“The materials we chose include very small amounts of things like magnesium and silicon, which are recommended parts of the daily diet,” says Rogers. When they dissolve, their concentrations are so low that they are hard to track against the background of the same substances already in an animal’s body.
Next, the team will test their sensors in pigs, and carry out further studies to convince regulators that the devices are completely safe. If things go well, Murphy hopes to start clinical trials in human patients within three to five years.
In the meantime, Rogers wants to make improvements, especially around power and connectivity. Currently, the sensor is wired to a secondary implant placed under the skin in a less vulnerable part of the body. The implant then wirelessly transmits the sensor’s data to an external source, while also receiving wireless power. The sensor and its wires will disappear completely, but for now, the secondary implant is only 85 percent degradable. Rogers thinks he can reach 100 percent.
“We think we can get it to 100 percent,” says Rogers.
“There is a huge unmet need to develop implantable devices that can achieve continuous monitoring of the body, to aid clinical decision making and ultimately improve patient quality of life,” says Jeff Karp from Brigham and Women's Hospital.
Although Rogers, Murphy, and their colleagues have risen to that challenge, "it will be important to determine how long the system can work for and how to calibrate measurements with changes in the biological response to the implanted materials."
Indeed, Rogers wants to make the sensor far more durable. Currently, it persists for a few days at most.
“We want to overengineer it so it lasts longer than what physicians want. We want four to five weeks, and we're not there yet.”
His team have also adapted their sensors to measure temperature, pH, and flow rates, as well as pressure. They should also be able to tweak the devices to detect specific molecules. And they could be implanted into many other body parts, like the heart or abdominal cavity.
Rogers is also moving from detecting to delivering. For example, he's working on dissolvable electric stimulators for treating nervous injuries.
“If you can stimulate a peripheral nerve in the right way, you can stimulate the healing process,” he says. “And after everything has healed, you don't need to go back in to do an extraction. We're quite far along the path for that.”
(Image via agsandrew/Shutterstock.com)
NEXT STORY: Government data is 'weird'





